Luminescent Glasses and Glass Ceramics for White Light Emitting Diodes by the Fraunhofer Institute and the South Westphalia University of Applied Science
Luminescent glasses or glass ceramics represent an interesting alternative to LED phosphor due to their high thermal and chemical stability. A series of rare-earth doped glasses are investigated for their potential application as photon converters for solid-state lighting applications. Franziska Steudel, Sebastian Loos, Bernd Ahrens, and Stefan Schweizer from the Fraunhofer Institute and the South Westphalia University of Applied Science respectively, show that the color coordinate of double-doped glass can be varied over a broad spectral range by changing the rare-earth doping ratio accordingly. In addition, double-doping allows for a change in the color coordinate by using different excitation wavelengths.
Glass is very versatile and a good host for rare-earth (RE) ions; it provides high optical transparency, good RE ion solubility, and can be cast in almost any shape or size. Luminescent glasses have attracted much attention in the last decades, in particular for lasers, optical fibers, and optical amplifiers [1]. The influence of the glass host on the optical properties of the embedded RE ions is weak, since the incompletely filled 4f shell of the RE is shielded by the surrounding 5s2 and 5p6 orbitals [2]. The position of the RE energy levels is similar for different matrix materials [3], whereas the intensity of the transitions varies with the crystal field and the phonon frequency of the host [4].
Matrix materials with low phonon energies are preferred, to avoid non-radiative relaxation, in particular, for high-efficiency applications. Borate glass is a suitable optical material with high mechanical, chemical and thermal stability [1, 5]. A widespread interest in borate glass is recorded by multiple publications on spectroscopy of RE ions in borate glasses in 2014 [6−11]. Compared to crystalline matrix materials, borate glass has a relatively high non-radiative transition rate for transitions within the 4f shell where one phonon or two phonons are involved, but for more than two phonons, the situation is reversed [12, 13]. The maximum phonon frequency of borate glass is 1,400 cm−1 and thus higher than for other glasses, e.g. 1,200 cm−1 for phosphate and 1,100 cm−1 for silicate glass [14].
The RE ions Eu3+ and Tb3+ have a great technological relevance. Their red and green luminescence is used in cathode ray tubes, fluorescent lamps, and plasma displays and have therefore been intensively studied. In addition, Tb3+ was found to be a good sensitizer to enhance luminescence efficiency of Eu3+ via energy transfer [15].
In the last years, Eu3+ and Tb3+ owned increased interest as a phosphor for white LEDs. Traditional white LEDs combine a blue LED chip and a yellow YAG:Ce3+ phosphor [16]. However, this method has a low color-rendering index and high color temperature due to a lack of red emission. For white light generation, an Eu3+ and Tb3+ doped phosphor can either be combined with a blue LED chip or with a third RE ion emitting in the blue such as Eu2+ [17, 18], Ce3+ [19, 20], or Tm3+ [21, 22] excited with an ultraviolet (UV) LED chip. With a three-color phosphor the light output is very low due to the lower efficiency of a UV LED compared to a blue LED [23].
In this work, Eu3+ and Tb3+ double-doped borate glass is investigated for its potential as a photon converter. Changing the Eu3+-to-Tb3+ ratio enables a continuous shift within the green to red spectral range. In addition, double-doping also allows for a change in the color coordinate by using different excitation wavelengths: A fast switch from green over yellow to red color coordinate is possible by choosing the excitation wavelength accordingly.
Experimental Details
Borate glasses using barium oxide as a network modifier are prepared. A ratio of two moles of boron oxide (B2O3) and one mole of barium oxide (BaO) is used. In this ratio the glass network consists of the highest possible amount of four-coordinated boron [24]. The glasses are additionally doped with Eu2O3 and Tb2O₃ for optical activation. The nominal chemical compositions of the samples are summarized in Table 1.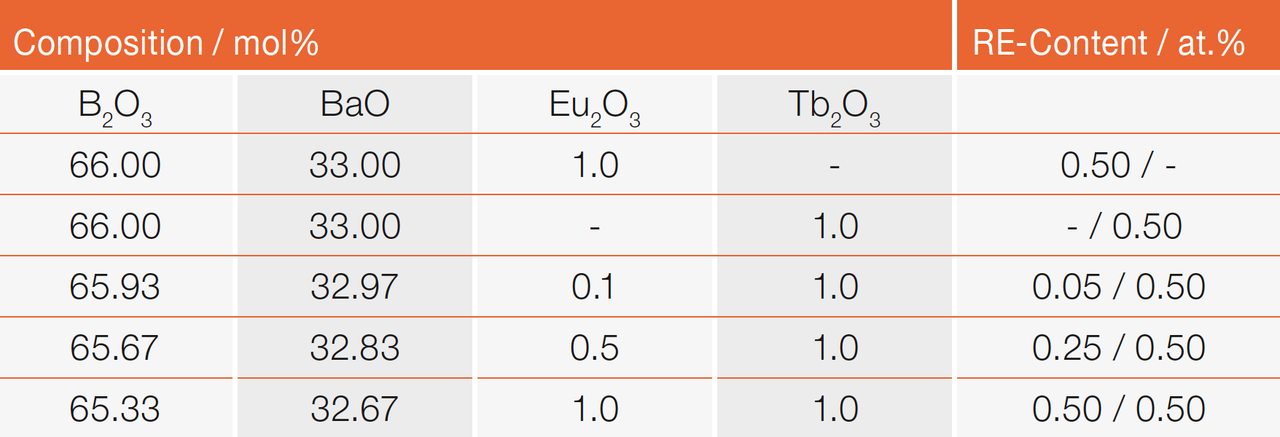
Table 1: Nominal composition of the borate glasses investigated
The chemicals are weighed in a platinum gold crucible (Pt/Au 95/5) and melted at 1100 °C for approximately 3 h. The melt is then poured onto a brass block at 500 °C which is below the glass transition temperature of barium borate glasses of Tg = 605 °C [25]. The glass is kept at this temperature for 3 h to eliminate residual mechanical and thermal stresses before being slowly cooled to room temperature.
Absolute photoluminescence (PL) quantum efficiency measurements are performed with a commercial quantum yields measurement system (Hamamatsu C9920-02G) coupled to a 3.3 inch integrating sphere with a xenon lamp (150 W) as excitation source and a photonic multichannel analyzer (PMA 12) as detector. The quantum efficiency is determined from the emission spectra in the spectral range from 470 nm to 900 nm.
Temperature-dependent PL measurements are performed with a custom-built fluorospectrometer (S&I Spectroscopy & Imaging GmbH FluoroVista) comprising a xenon lamp (75 W) coupled to a 300 mm focal length monochromator (Princeton Instruments Acton 2300) for excitation and a peltier-cooled photomultiplier (Hamamatsu R943-02) coupled to a second 300 mm focal length monochromator (Princeton Instruments Acton 2300) for detection.
Results and Discussion
Rare-earth ion luminescence and quantum efficiency of single-doped borate glass
Though the spectral positions of RE ion emission are well known [2, 3, 26], the transition intensity ratios vary due to differences in crystallinity and phonon frequencies of the host material. The emission spectra of Eu3+ and Tb3+ single-doped borate glasses are shown in Figure 1. Upon excitation at 393 nm, the Eu3+ doped glass (Figure 1a) shows the typical Eu3+ emissions in the red spectral range, which are caused by transitions from the 5D0 state to the ground state levels 7F0 (580 nm), 7F1 (592 nm), 7F2 (611 nm), 7F3 (652 nm), and 7F4 (700 nm). The electric-dipole transition 5D0 to 7F2 is hypersensitive to variations in crystal symmetry [2]. The high intensity of this transition in borate glass indicates the amorphous nature of the matrix material with low inversion symmetry for the Eu3+ ion. In Figure 1b, the typical Tb3+-related emissions at 490 nm, 543 nm, 583 nm, and 622 nm can be assigned to transitions from the 5D4 excited state to the ground state levels 7FJ (J = 6, 5, 4, and 3), respectively.
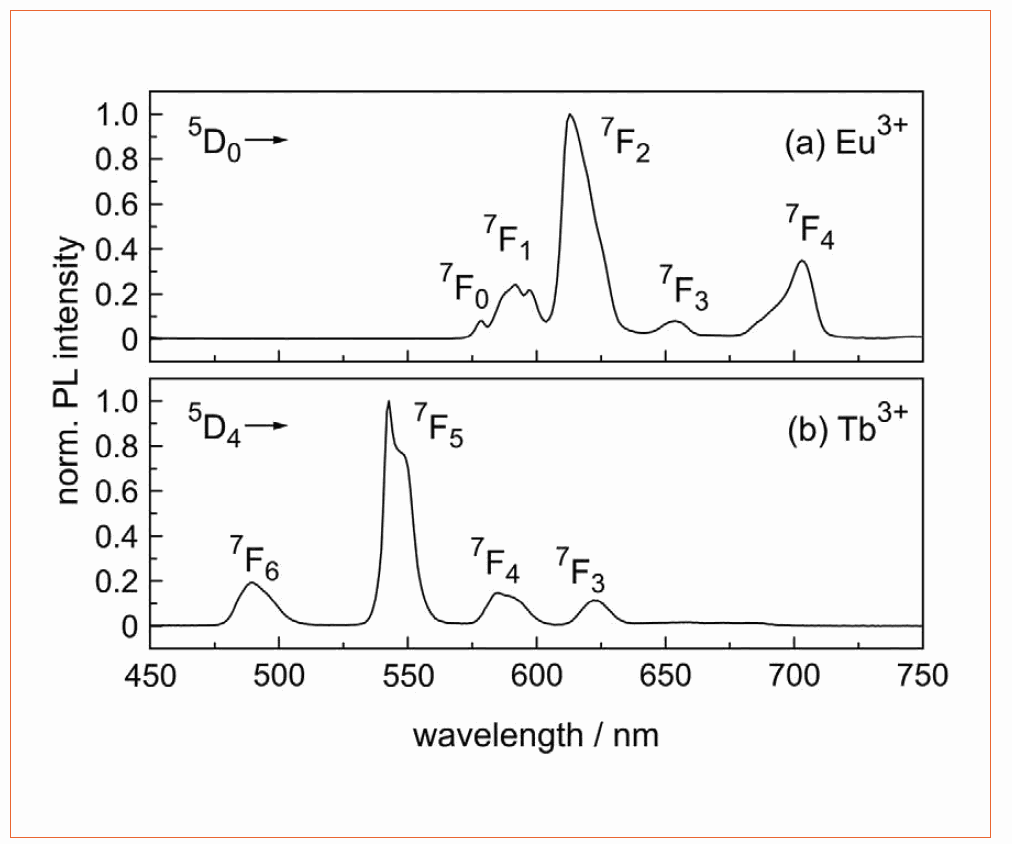
Figure 1: Normalized emission spectra of borate glass doped with (a) Eu3+ and (b) Tb3+. The Eu3+ excitation was carried out at 393 nm, Tb3+ was excited at 370 nm. The emission transitions are indicated
For both RE ions, emissions in the blue spectral range (from higher energy levels) are quenched due to the high maximum phonon frequency of 1,400 cm−1 in borate glass [14]. However, the maximum quantum efficiency of the transitions observed is up to 86 %. Figure 2a shows the spectrally-resolved quantum efficiency of a 0.5 at.% Eu3+ doped borate glass: The transitions at 393 nm, 484 nm, and 530 nm provide quantum efficiency values higher than 80 %. For the 0.5 at.% Tb3+ doped glass (Figure 2b), the QE has its maximum at 464 nm with approx. 63 %. For wavelengths shorter than 350 nm, the QE is significantly reduced due to base glass absorption.
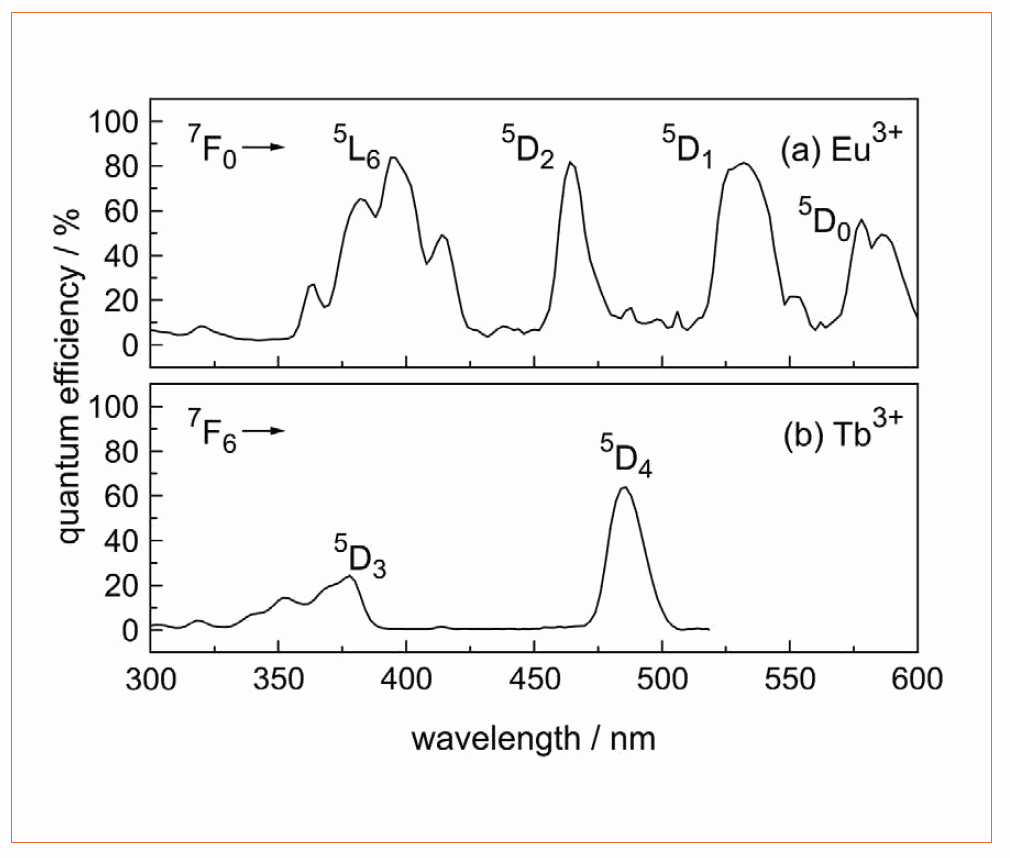 Figure 2: Quantum efficiency spectra of borate glass doped with (a) Eu3+ and (b) Tb3+. The main transitions are indicated
Figure 2: Quantum efficiency spectra of borate glass doped with (a) Eu3+ and (b) Tb3+. The main transitions are indicated
Temperature stability
For white LEDs, the temperature stability of the phosphor on top is of vital importance. During operation, today’s LEDs reach junction temperatures of 150°C and even 200°C in case of high-power LEDs. The high junction temperatures lead to a significant change in color appearance [27] since the phosphor emission intensity decreases with increasing temperature. There is a clear need for color-stable phosphors.
Figure 3 shows the temperature-dependent photoluminescence intensity of Eu3+ and Tb3+ single-doped glasses. Each emission spectrum is integrated and normalized to the value at room temperature. At a temperature of 275°C, the intensity for Eu3+ and Tb3+ doping decreases to 58 % and 93 %, respectively. For both RE ions, the so-called quenching temperature, i.e. the temperature at which the intensity amounts to 50 % of its initial intensity, is thus approximately 300°C for Eu3+ and even significantly higher for Tb3+. For comparison, the quenching-temperature of the conventional LED phosphor YAG:Ce3+ lies at 270°C [28]. Although the intensity of the spectra decreases slightly, the spectral behavior does not change, i.e. the color appearance is stable in the investigated temperature range [29].
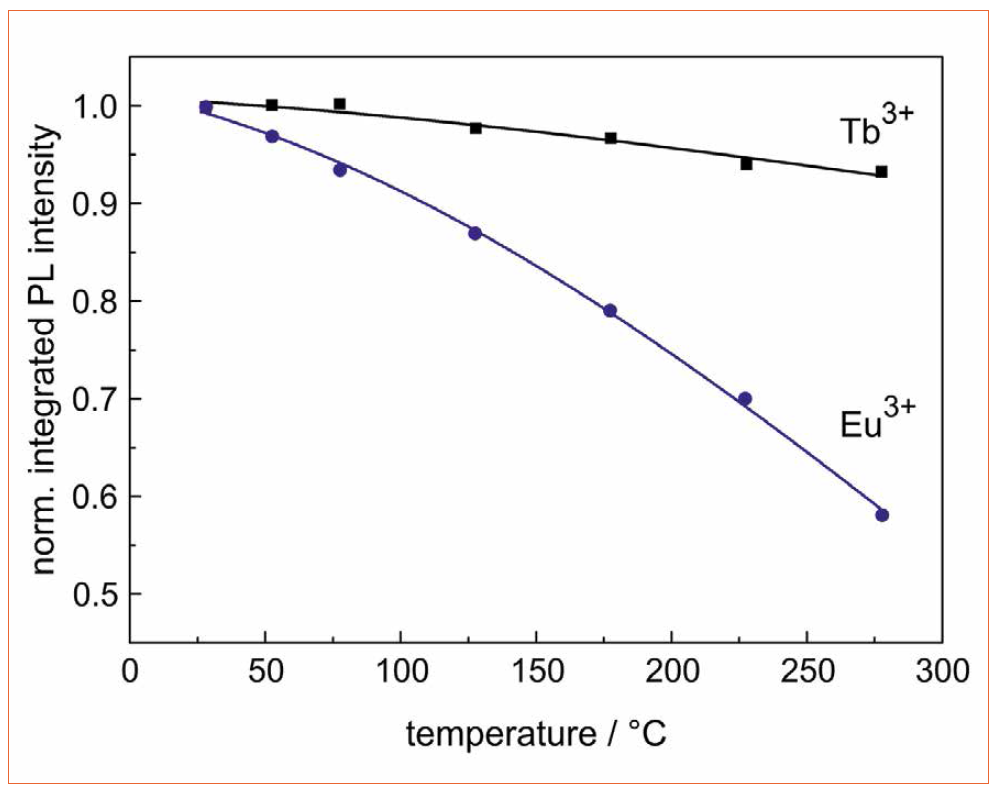
Figure 3: Temperature-dependent normalized photoluminescence intensity of Eu3+ and Tb3+ singledoped borate glass, recorded under 376 nm and 395 nm excitation, respectively
Color mixing for solid state lighting with double-doped glass
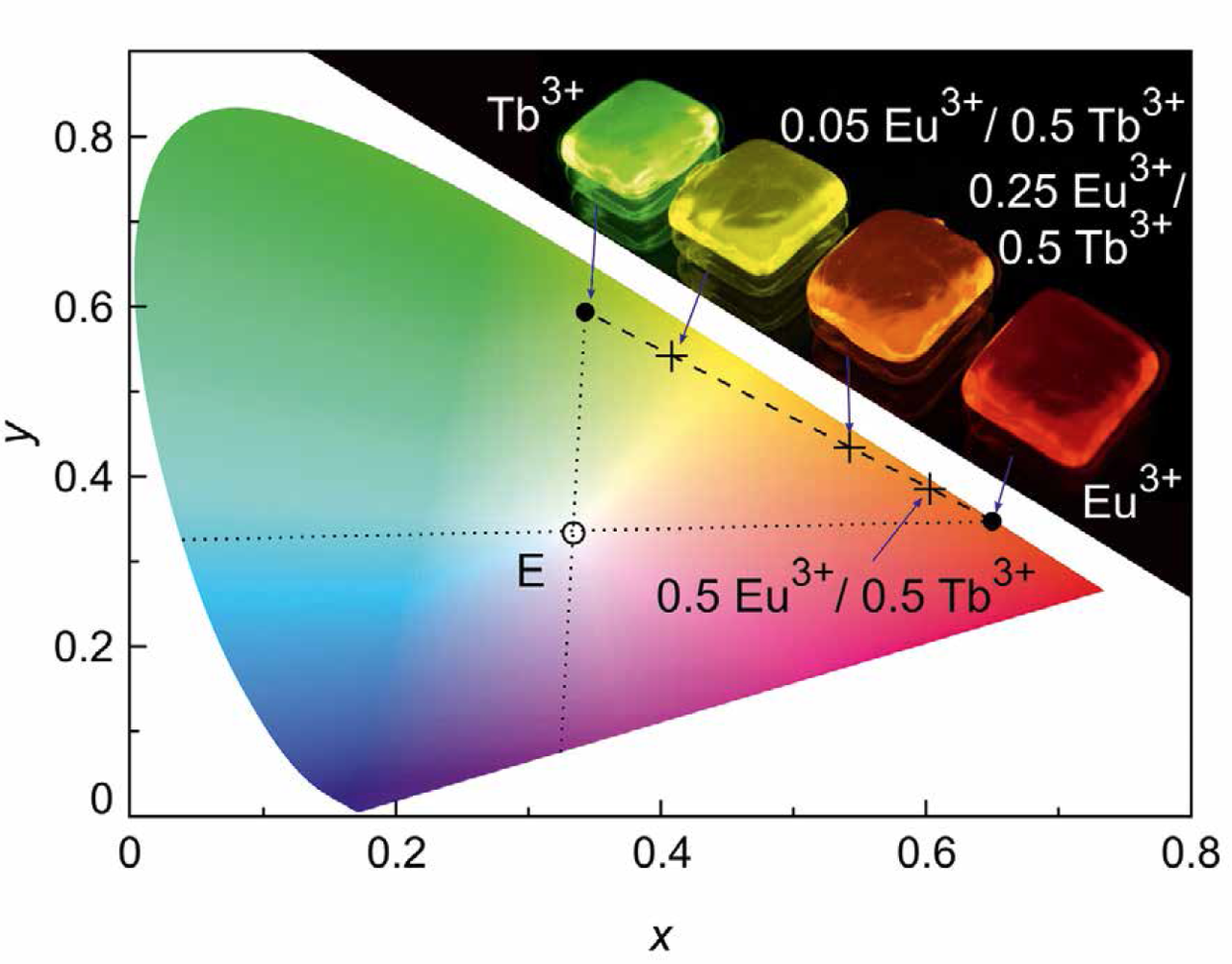
Figure 4 shows the color coordinates of the Eu3+ and Tb3+ single-doped borate glasses in the CIE (Commission internationale de l’eclairage) color space chromaticity diagram determined from the corresponding emission spectra (Figure 1). Both RE ions are close to the edge of the CIE chromaticity diagram. They provide high color saturation since the blue emissions are suppressed by the high phonon frequencies of the borate host. Compared to Eu3+, the Tb3+ emission is slightly shifted to the blue because of the 5D4 to 7F6 transition at 490 nm.
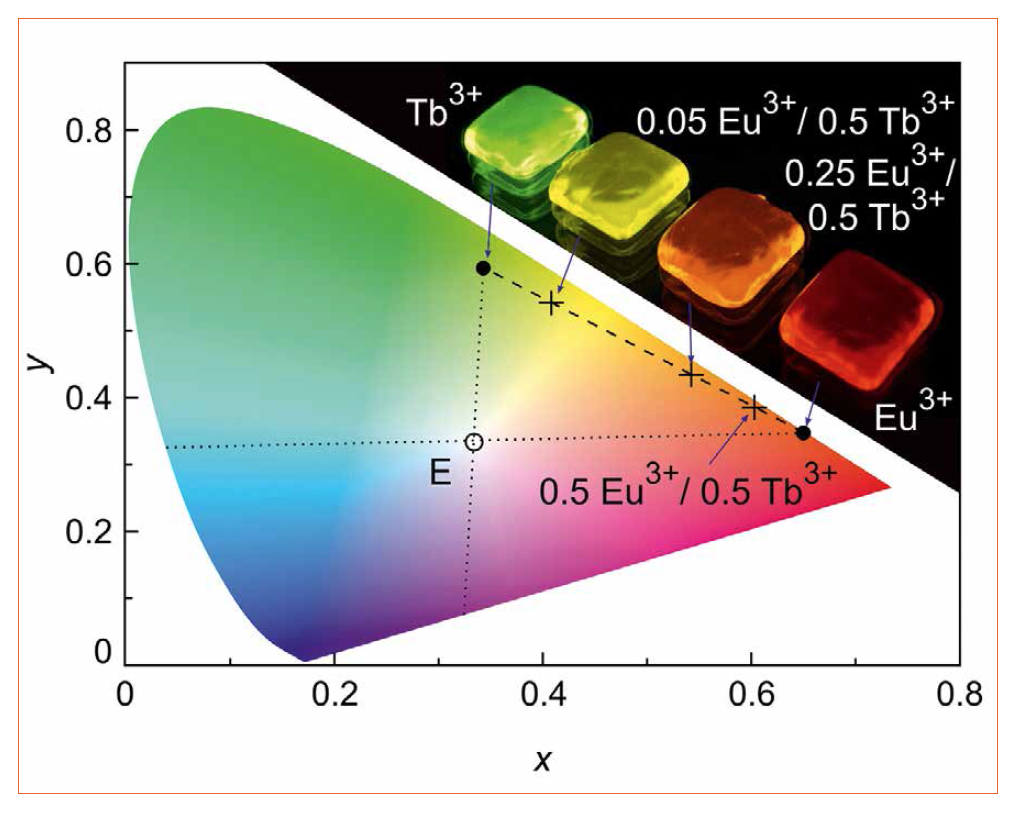
Figure 4: CIE color space chromaticity diagram with point of equal energy, E, and the color coordinates for the Eu3+ and Tb3+ single- (full dots) and double-doped (crosses) borate glasses under 380 nm excitation. The dotted lines indicate the complementary blue part. The inset shows our of the five glasses under UV illumination
The emission spectra of the double-doped glasses depend significantly on the RE ion concentration (Figure 4). The color coordinate of the Eu3+ / Tb3+ double-doped glass shifts from the green (Tb3+) to the red (Eu3+) spectral range with increasing Eu3+ concentration. The dashed line connecting the color coordinates of the Eu3+ and Tb3+ single-doped glasses indicates all possible color coordinates of the double-doped glass. Note that the emission spectrum of the equally double-doped glass (see 0.5 at.% Eu3+ / 0.5 at.% Tb3+ in Figure 4) is shifted to the red spectral range since there is a significant energy transfer from Tb3+ to Eu3+ [15, 30]. The dotted lines going through the point of equal energy, E, indicate the complementary blue region. This method of color mixing by varying the RE ratio is a conventional method, in particular used for white LED phosphors. However, the color of the phosphor is then fixed and cannot be changed during use.
In addition to the above-described color mixing by changing the RE doping concentration, double-doped borate glass offers the opportunity to vary the color coordinates upon changing the excitation wavelength accordingly. This is shown in Figure 5 for the 0.05 at.% Eu3+ / 0.5 at.% Tb3+ doped glass. For UV excitation at 350 nm, the Tb3+ emission dominates the spectrum resulting in a green color appearance, while 380 nm excitation leads to a yellow one. Excitation at 385 nm and 390 nm results in an orange and red color impression, respectively, due to the strong Eu3+ absorption bands at these wavelengths. A color change during use is thus possible. Changing the excitation wavelength, e.g. from 350 nm to 390 nm, the color coordinates switch from green to red.
Using different excitation wavelengths enables an immediate change in color coordinates with one phosphor. This method might be used in control lamps to attract attention or in lighting applications where a change in color coordinates during use is wanted.
Conclusion
Eu3+ / Tb3+ double-doped borate glass has been investigated for its potential as a photon converter for use in white LEDs. With the Tb3+ emission in the green and the Eu3+ emission in the red spectral range, color mixing from green to red is enabled by varying the ratio of the RE doping level accordingly. In addition, using different excitation wavelengths can also vary the color coordinates of the phosphor. Switching between different excitation wavelengths enables a fast change in color coordinates from green to yellow then to red. Moreover, the investigated glasses provide good temperature stability in the temperature range investigated.
Acknowledgements:
The Fraunhofer Application Center for Inorganic Phosphors in Soest is supported by the “Ministerium für Innovation, Wissenschaft und Forschung des Landes Nordrhein-Westfalen”.
References:
[1] H. Lin, E. Y.-B. Pun, X. Wang, X. Liu, J. Alloys Compd. 2005, 390, 197-201
[2] G. Blasse, B. C. Grabmaier, Luminescent Materials, Springer Verlag, Berlin Heidelberg 1994
[3] G. H. Dieke, H. M. Crosswhite, Appl. Opt. 1963, 2, 675-686
[4] M. Dejneka, E. Snitzer, R. Riman, J. Lumin. 1995, 65, 227-245
[5] N. Soga, K. Hirao, M. Yoshimoto, H. Yamamoto, J. Appl. Phys. 1988, 63, 4451-4454
[6] J. Hu, X. Gong, Y. Chen, J. Huang, Y. Lin, Z Luo, Y. Huang, Opt. Mater. 2014, 38, 108-112
[7] T. Y. Lim, H. Wagiran, R. Hussin, S. Hashim, M. Saeed, Physica B 2014, 451, 63-67
[8] M. Mhareb, S. Hashim, S. Ghoshal, Y. Alajerami, M. Saleh, R. Dawaud, N. Razak, S. Azizan, Opt. Mater. 2014, 37, 391-397
[9] J. Pisarska, A. Kos, W. A. Pisarski, Spectrochim. Acta Part A 2014, 129, 649-653
[10] K. Swapna, S. Mahamuda, A. S. Rao, M. Jayasimhadri, S. Shakya, G. V: Prakash, J. Lumin. 2014, 156, 180-187
[11] H. Xiong, L. Shen, E. Pun, H. Lin, J. Lumin. 2014, 153, 227-232
[12] J. M. F. van Dijk, M. F. H. Schuurmans, J. Chem. Phys. 1983, 78, 5317-5323
[13] M. F. H. Schuurmans, J. M. F. van Dijk, J. M. F., Physica B 1984, 123, 131-155
[14] M. Shojiya, M. Takahashi, R. Kanno, Y. Kawamoto, K. Kadono, J. Appl. Phys. 2007, 82, 6259-6266
[15] L. G. Van Uitert, R. R. Soden, J. Chem. Phys. 1962, 36, 1289-129
[16] P. Schlotter, R. Schmidt und J. Schneider, Appl. Phys. A 1997, 64, 417-418
[17] X. Chen, J. Zhao, L. Yu, C. Rong, C. Li, S. Lian, J. Lumin. 2011, 131, 2697-2702
[18] Y. Zhang, Z. Zhu, Y. Qiao, Mater. Lett., 2013, 93, 9-11
[19] U. Caldiño, E. Álvarez, A. Speghini, M. Bettinelli, J. Lumin. 2013, 135, 216-220
[20] X. Zhang, L. Zhou, Q. Pang, M. Gong, Opt. Mater. 2014, 36, 1112-1118
[21] V. H. Romero, E. De la Rosa, T. López-Luke P. Salas, C. Angeles-Chavez, J. Phys. D: Appl. Phys. 2010, 43, 465105
[22] C.-j. Zhao, J.-l. Cai, R.-y. Li, S.-l. Tie, X. Wan. J.-y. Shen, J. Non-Cryst. Solids 2012, 358, 604-608
[23] Y. Muramoto, M. Kimura, S. Nouda, Semicond. Sci. Technol. 2014, 29, 084004
[24] W. Müller-Warmuth and H. Eckert, Phys. Reports, 1982, 88, 91
[25] S. Kapoor, H. B. George, A. Betzen, M. Affatigato, S. Feller, J. Non-Cryst. Solids 2000, 270, 215-222.
[26] K. Binnemans, R. V. Deun, C. Görller-Walrand, J. L. Adam, J. Non-Cryst. Solids 1998, 238, 11-29
[27] X. Yang, J. Liu, H. Yang, X. Yu, Y. Guo, Y. Zhou, and J. Liu, J. Mater. Chem. 2009, 19, 3771-3774
[28] R.-Y. Yang, C.-T. Pan, K.-H. Chen, C.-Y. Hung, Opt. Mater 2013, 35, 2183-2187
[29] S. Loos, F. Steudel, B. Ahrens, R.L. Leonard, J.A. Johnson, S. Schweizer, Phys. Status Solidi C, submitted on 15 June 2015
[30] J. Pisarska, A. Kos, M. Soltys, L. Zur, W. A. Pisarski, J. Non-Cryst. Solids 2014, 388, 1-5