LED and Lens Degradation Through Volatile Organic Compounds
In general, LEDs are very resistant light sources. Nevertheless, glues, conformal coatings, o-rings, gaskets, and potting compounds, all materials that are frequently used in the construction of LED based luminaires or lamps and often contain volatile organic compounds (VOCs) may have a negative impact on performance, reliability and lifetime. Edward Steinke, application engineer at Cree, explains the set of problems and how to prevent these issues based on Cree’s products, using their test kit components, as well as components for lamp and luminaire applications.
The presence of volatile organic compounds (VOCs) in LED-based solid-state lighting (SSL) designs can impair the performance and reduce the lifetime of these illumination systems. Glues, conformal coatings, O-rings, gaskets and potting compounds are materials frequently used in the construction of LED based luminaires or lamps and often contain VOCs. The presence of chemically incompatible VOCs on or near LEDs can degrade the light output levels or cause changes in the chromaticity point of the light. Chemical incompatibility in SSL is often a localized phenomenon, occurring in luminaire designs that seal portions of the system, resulting in an LED’s operating environment at elevated temperatures with little or no air movement. However, proper design and adequate testing can prevent chemical incompatibility effects. Most SSL luminiare types use a blue light LED chip covered with a yellow phosphor and encapsulant for converting the blue light to a broader white light spectrum. A silicone-based lens covers this whole LED assembly.
The unique silicone materials utilized in LEDs have excellent light transmittance characteristics: stable over wide temperature ranges, resistant to yellowing due to ultraviolet (UV) exposure and easily molded. This results in a high performance yet cost-effective LED. The basic structure of the LED’s lens and encapsulants is a silicone polymer, which is a stable chemical compound.
Any VOCs present in a SSL system can diffuse into the gas permeable silicone lens and encapsulants of the LED. Within the molecular structure of these silicone materials, the VOCs will occupy a free space in the interwoven silicone polymer. With subsequent exposure to high photon energy emitted from the LED, along with the heat from the lighting system and the environment, the volatile compounds trapped in the LED’s lens or encapsulants can discolor. This discoloration of the trapped VOCs can degrade the light emitted from the LED. This discoloration tends to occur in blue, royal blue and white light producing LEDs that use blue wavelength LED chips with yellow phosphors for spectrum conversion. This sensitivity to VOC is not unique to one LED manufacturer but is a known problem for all types of blue, royal blue and white light LEDs. Chemically induced discoloration is less prevalent and not as noticeable with amber, red or green LEDs since these color LEDs have longer wavelengths, therefore a lower frequency, and produce lower photonic energy compared to blue LEDs. Photonic energy (E) is defined by the Planck- Einstein equation of E = hf, where h is Planck’s constant and f is frequency, thus a higher frequency produces a higher photonic energy.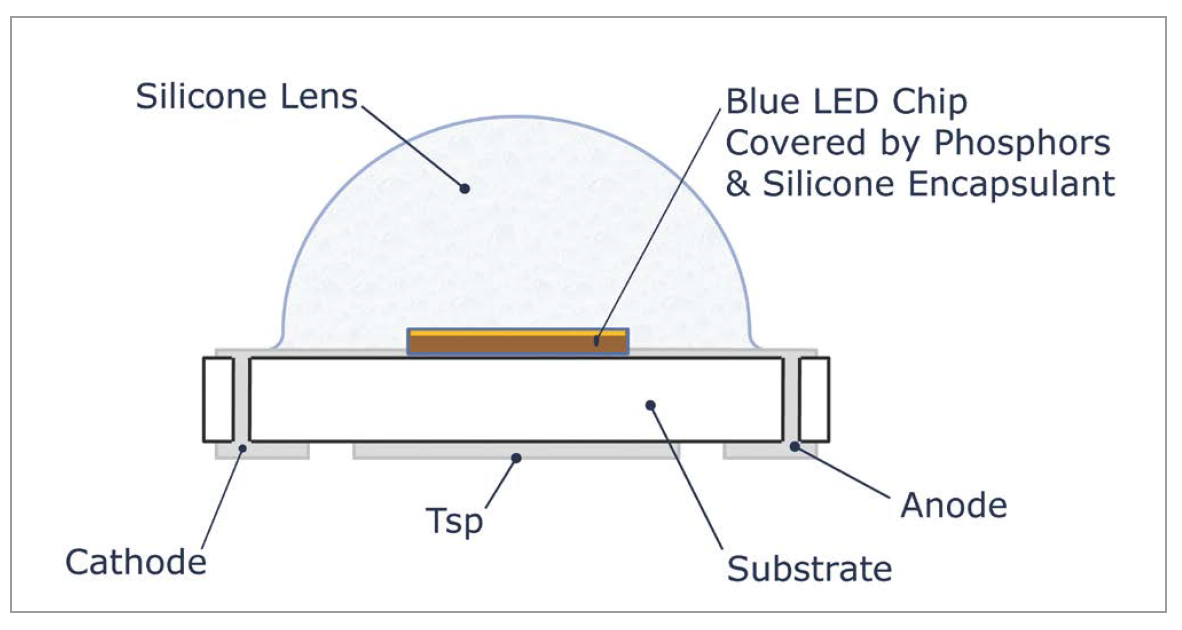 Figure 1: Cross-sectional structure of a typical lighting-class LED
Figure 1: Cross-sectional structure of a typical lighting-class LED
The Effects of Chemicals
Chemicals utilized in manufacturing SSL systems can result in LED light quality degradation or even complete luminaire failure. Figure 2 illustrates the degradation effect from a VOC on the silicone encapsulant that covers the LED chip.
 Figure 2: Examples of normal (left) and VOC degraded (right) LEDs
Figure 2: Examples of normal (left) and VOC degraded (right) LEDs
The photograph on the left shows the normal appearance of an LED. On the right, the same type XLamp chip has a pronounced brown discoloration due to exposure to VOCs while in operation with high photonic power output and at nominal environmental temperatures. This discoloration of the encapsulants is on the top of the LED chip, localized to the area just above the chip surface, closest to the source of heat and high photon energy. The VOCs occupying a free space within the lens or encapsulant typically do not cause permanent damage to the silicone material or the LED chip. In many instances, removing a secondary optic or otherwise venting the LED’s environment will allow it to clear and recover in just a few hours of operation. However, the discolored VOC in the silicone polymer will not clear if the LED’s operating environment remains saturated with the VOC.
Figure 3 shows graphic examples of this type of chemical degradation followed by out-gassing of the VOC, which clears up a darkened LED. In figure 3, photograph 1 is a VOC darkened LED caused by operation in an environment with incompatible VOCs. Photograph 2 is after 24 hours of operation in a VOC free environment and photograph 3 is after 48 hours without the presence of VOCs. Photograph 4 is after 72 hours of VOC-free operation and the LED is clear.
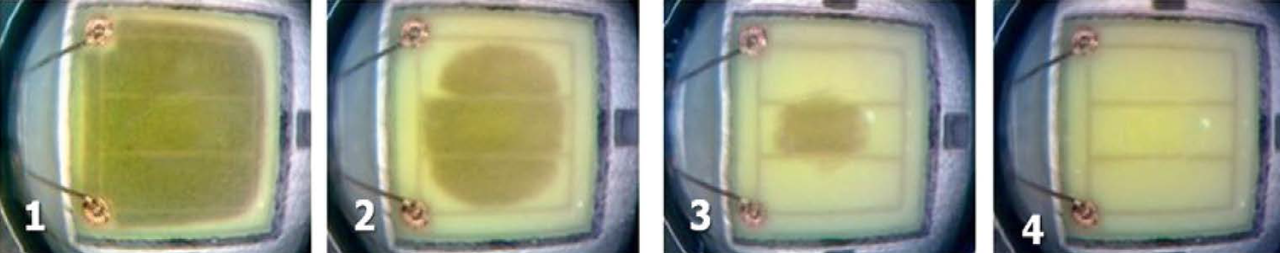 Figure 3: VOC degraded LEDs allowed to out-gas
Figure 3: VOC degraded LEDs allowed to out-gas
The application engineering team conducted a controlled chemical compatibility experiment to demonstrate and document the effect of this VOC reversibility on LEDs. Over an interval of 450 hours, three sets of 10 LEDs (30 LEDs in total) were first contaminated with a known high-VOC chemical and then for >450 hours tested for luminous flux output in three different test environments. One group was in an open-air environment, the second group in a sealed secondary optics environment and the third group started out with sealed optics, but later vented at 325 hours into the test.
The first group of ten LEDs operated in the open-air environment and despite the deliberate contamination showed no degradation from the initial luminous flux measurements of approximately 100 lumens. The sealed second group suffered a 90% loss of light output after 450 hours of operation. The third sample group of LEDs after 325 hours of operation also suffered a 90% loss of luminous flux output. Venting the enclosure of this third sample group allowed the VOCs to escape and 24 hours later, the third sample set of LEDs recovered virtually all of the lost luminous flux output. Figure 4 provides a graphical summary of the luminous flux output versus time for these three test cases.
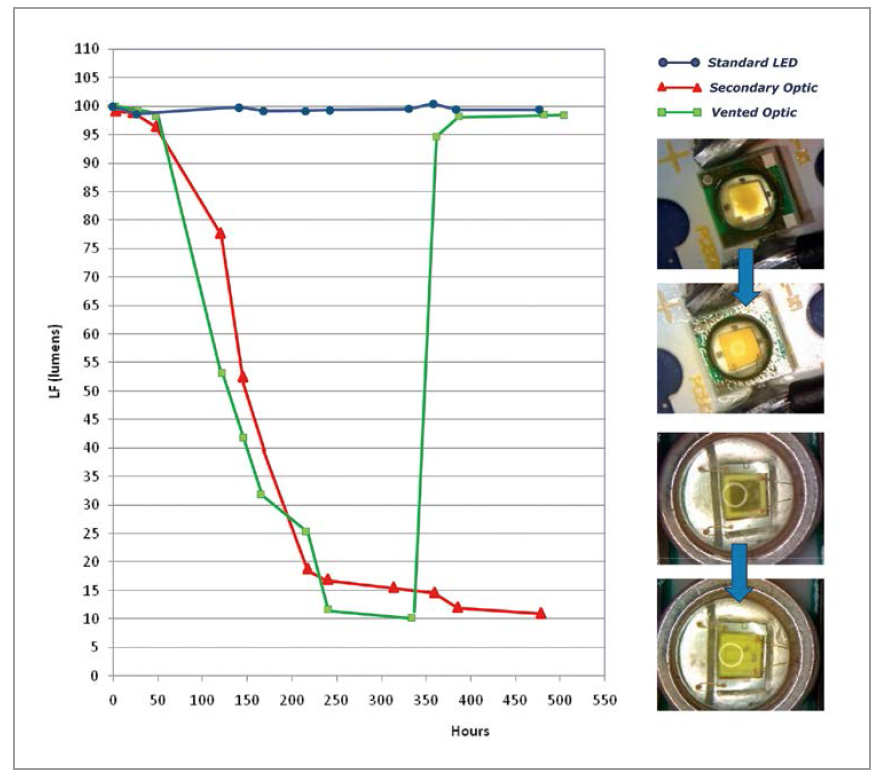 Figure 4: Test results for VOC in vented and unvented designs demonstrate the reversibility of VOC discoloration in vented optics
Figure 4: Test results for VOC in vented and unvented designs demonstrate the reversibility of VOC discoloration in vented optics
Chemical Compatibility Testing
Chemical compatibility testing validates that the chemicals or compounds within the SSL system are compatible with the LEDs utilized. The focus for this testing should be on gasket materials, adhesives, conformal coatings, soldering flux or any residual chemicals that may be in close contact to the LED lens and hermetically sealed with the LED.
A chemical compatibility test kit for various LED product lines helps to identify critical VCOs. The kits are complete with a metal-core circuit board populated with six LEDs and glass vials with adhesive to create an airtight test environment. Figure 5 is a photograph of a chemical compatibility test kit for XR-E devices with all the supplied test materials.
 Figure 5: Chemical compatibility test kits
Figure 5: Chemical compatibility test kits
The test kits utilize small sealed glass enclosures that test a single material or chemical sample for any effect on a single LED. The proper test setup is to place material under test on top of the first three LEDs on the board, then place the same test material at the base, away from the lens, of the next two LED components. The final LED will be the control reference and tests an LED without any sample material. An airtight seal, made with the Arctic Silver adhesive, around the diameter of the glass vials covers the LED and sample material.
Wires from a constant current supply connect to each chemical board. Drive current varies based on component type for these tests, 700 mA for the XP-E and XR-E LEDs and 350 mA for the MX-6 LEDs. Typically, testing is for about 1000 hours, monitoring for changes in light intensity or color or simply just visual appearance of the LED’s phosphor layer with the LED turned off. Test results indicating chemical compatibility issues often show up in 48 hours after initial illumination. Figure 6 shows a typical chemical compatibility test setup under operation for about 720 hours and it is easy to note the color shift in the LED third from the left and a significant dimmer LED, which is fifth from the left.
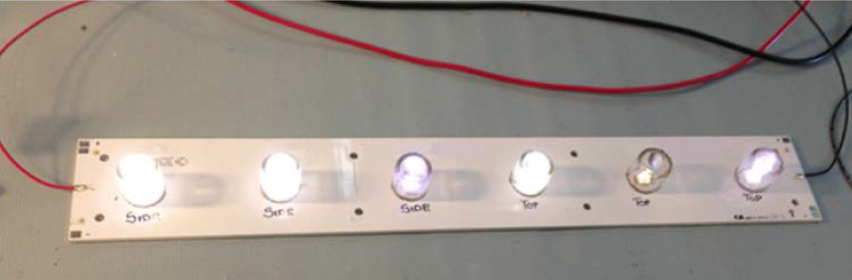 Figure 6: Chemical compatibility test card in operation
Figure 6: Chemical compatibility test card in operation
Summary of the Testing Results
Many common chemicals can outgas aromatic hydrocarbons, that are arenes, and fumes from even small amounts of these chemicals tend to discolor or damage the light output from LEDs. Table 1 contains a list of common chemicals that were found to be harmful to the tested LEDs, and therefore not recommended to be used in or around a LED based SSL system.
 Table 1: Common chemicals with known LED compatibility issues
Table 1: Common chemicals with known LED compatibility issues
Table 2 offers a brief list of circuit board conformal coating materials that showed to be harmless when tested.* However, do not apply conformal coatings directly on to the LED lens, as this may affect the LED optical performance and reliability.
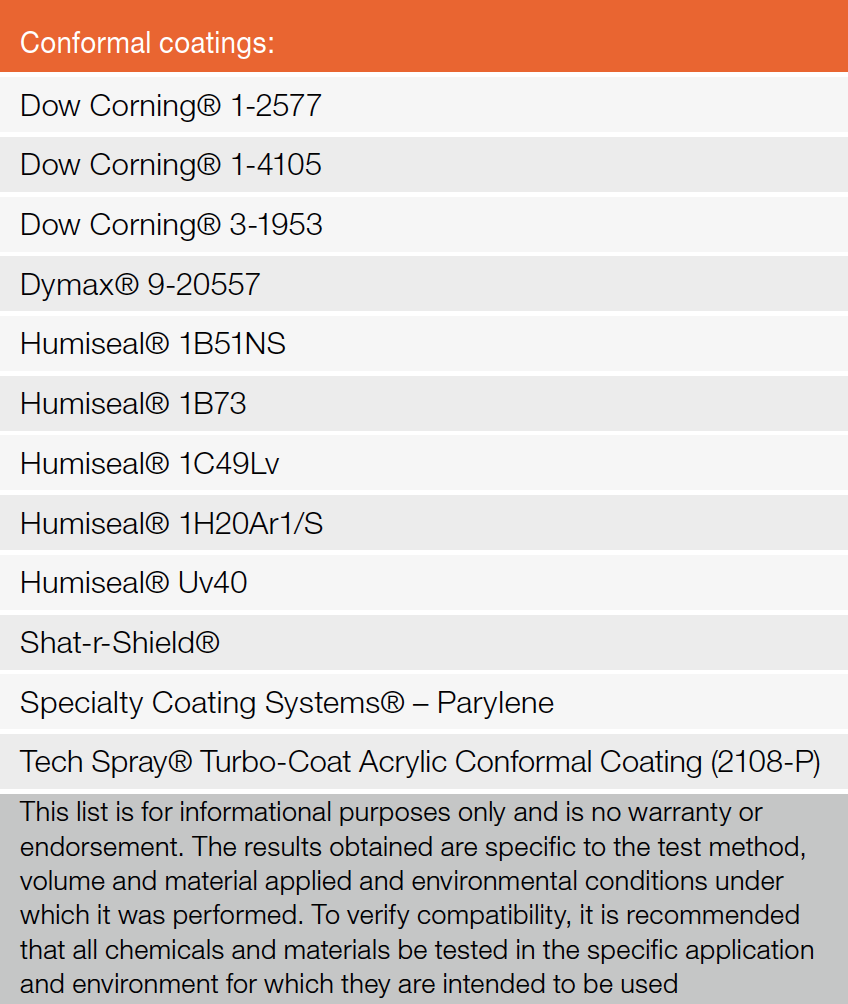 Table 2: Conformal coating compatible with XLamp LED
Table 2: Conformal coating compatible with XLamp LED
Compatibility test results obtained for common materials compounds are outlined in table 3. These test results are specific to the test method, volume of material applied and environmental conditions encountered during the testing. For new designs, all chemicals and materials utilized in a SSL product should be compatibility tested with the specific type of LED and under the environmental condition in which they will operate.
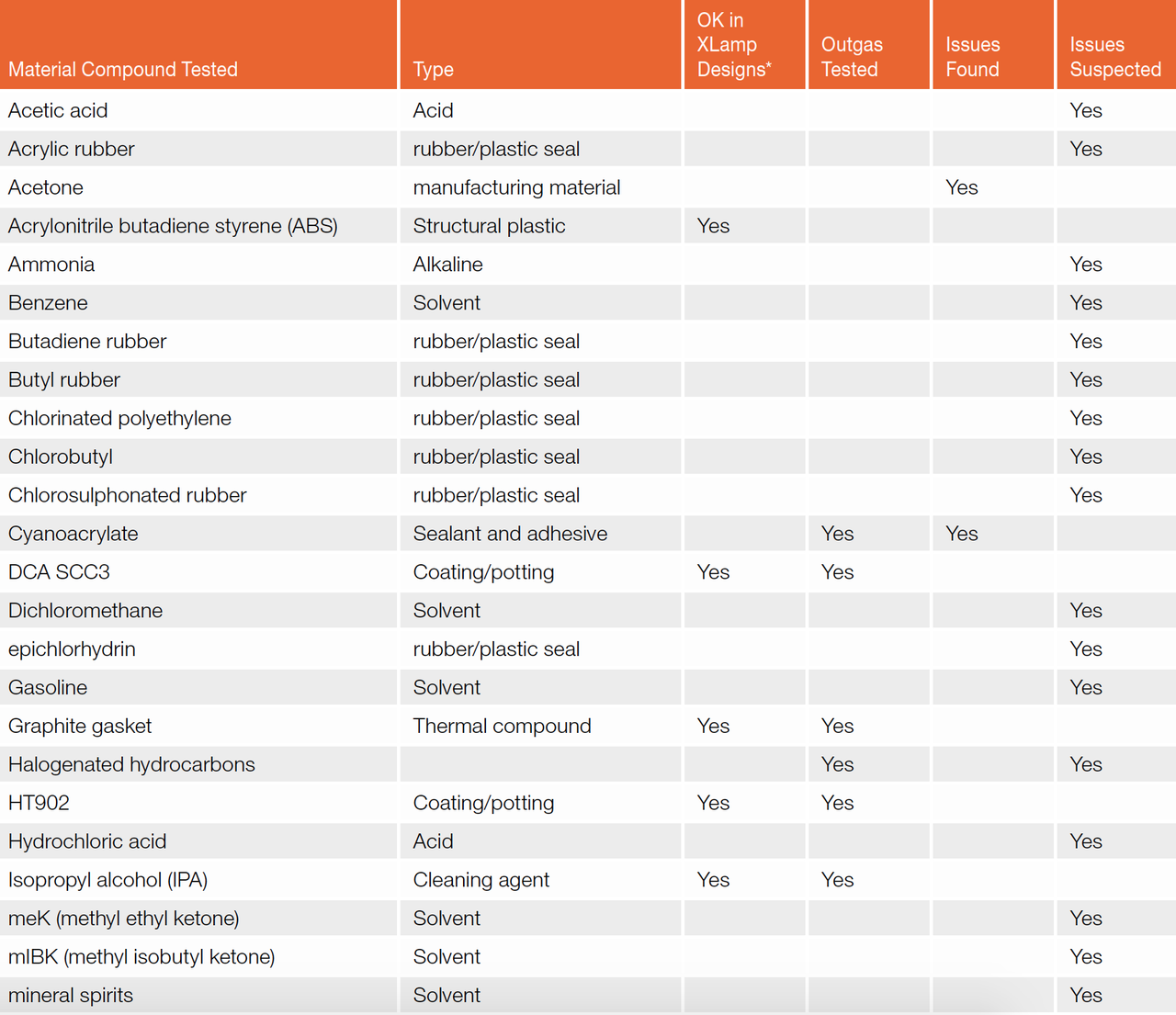
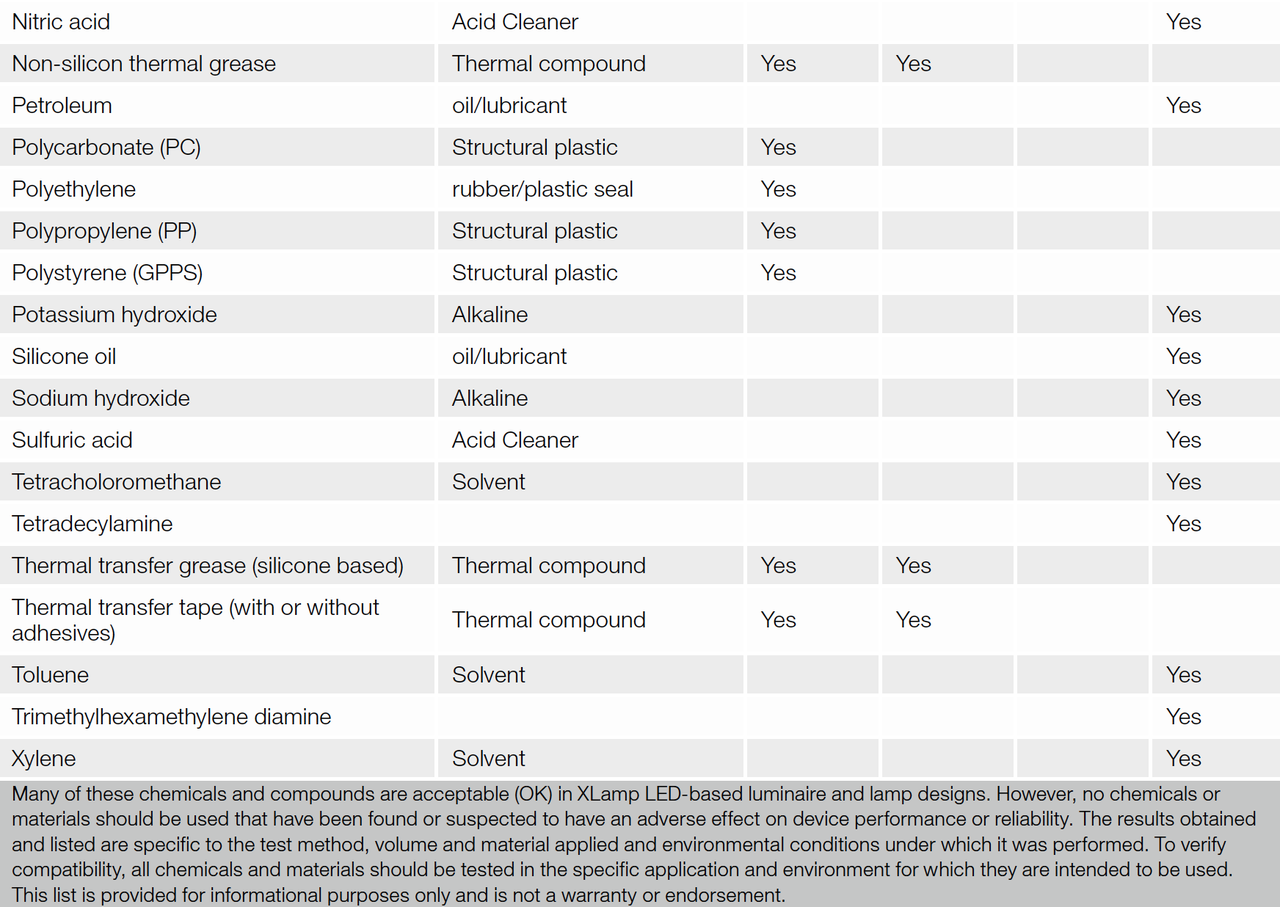
Table 3: Material compound compatibility list
To help speed up the adoption of LED based SSL, some LED providers started programs to help lighting manufacturers find complementary components from third-party manufacturers that work with their LED products, like the Cree Solution Provider Program (CSP). The CSP helps lighting manufacturers with their LED lighting design requirements and can enable a shorter design cycle to get products to market faster. As part of this CSP activity, Henkel, a major manufacturer of materials for use in electronics, has helped to develop a chemical compatibility reference guide for LEDs. This guide, based on test results from the chemical compatibility test kits, covers thread-lockers, general bonding, potting compounds, lens bonding and sealing products that have been found to be compatible with LEDs during testing.
In conclusion, VOCs emitted from materials used in the construction of LED based SSL systems can penetrate the silicone lenses and encapsulants of LEDs. These VOCs in the silicone can discolor when exposed to heat and high photonic energy of the LED. The result can produce significant loss of light output or color shift from the LED. Proper material selection for the SSL design and venting of the enclosure or secondary lens assemblies reduces the risk of chemical compatibility issues in SSL luminaire systems. Compatibility testing of the materials selected for the assembly of the SSL system can help ensure the long-term high performance of lighting-class LED designs.

